How it works
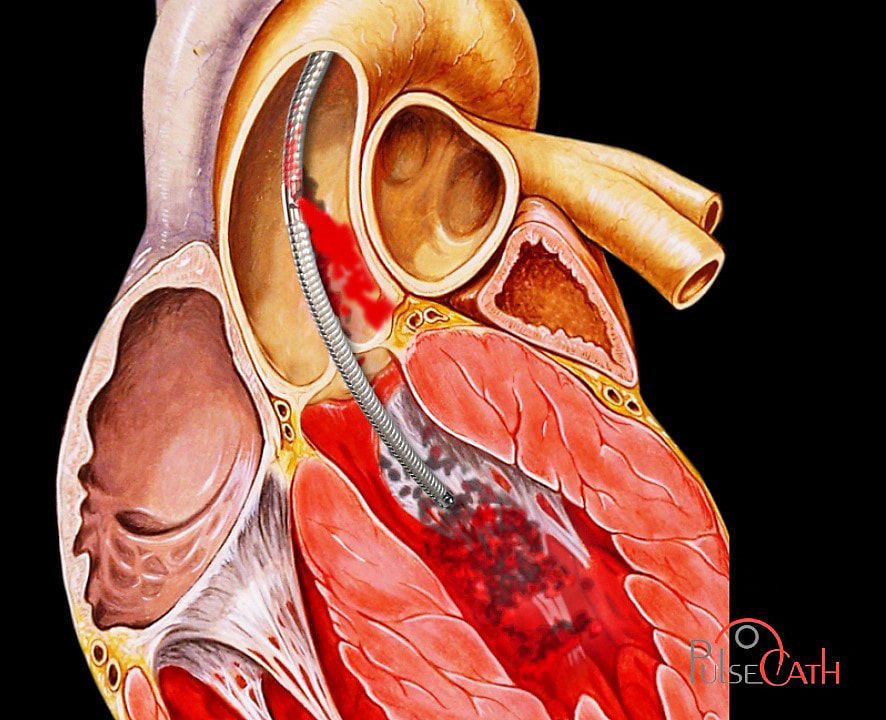
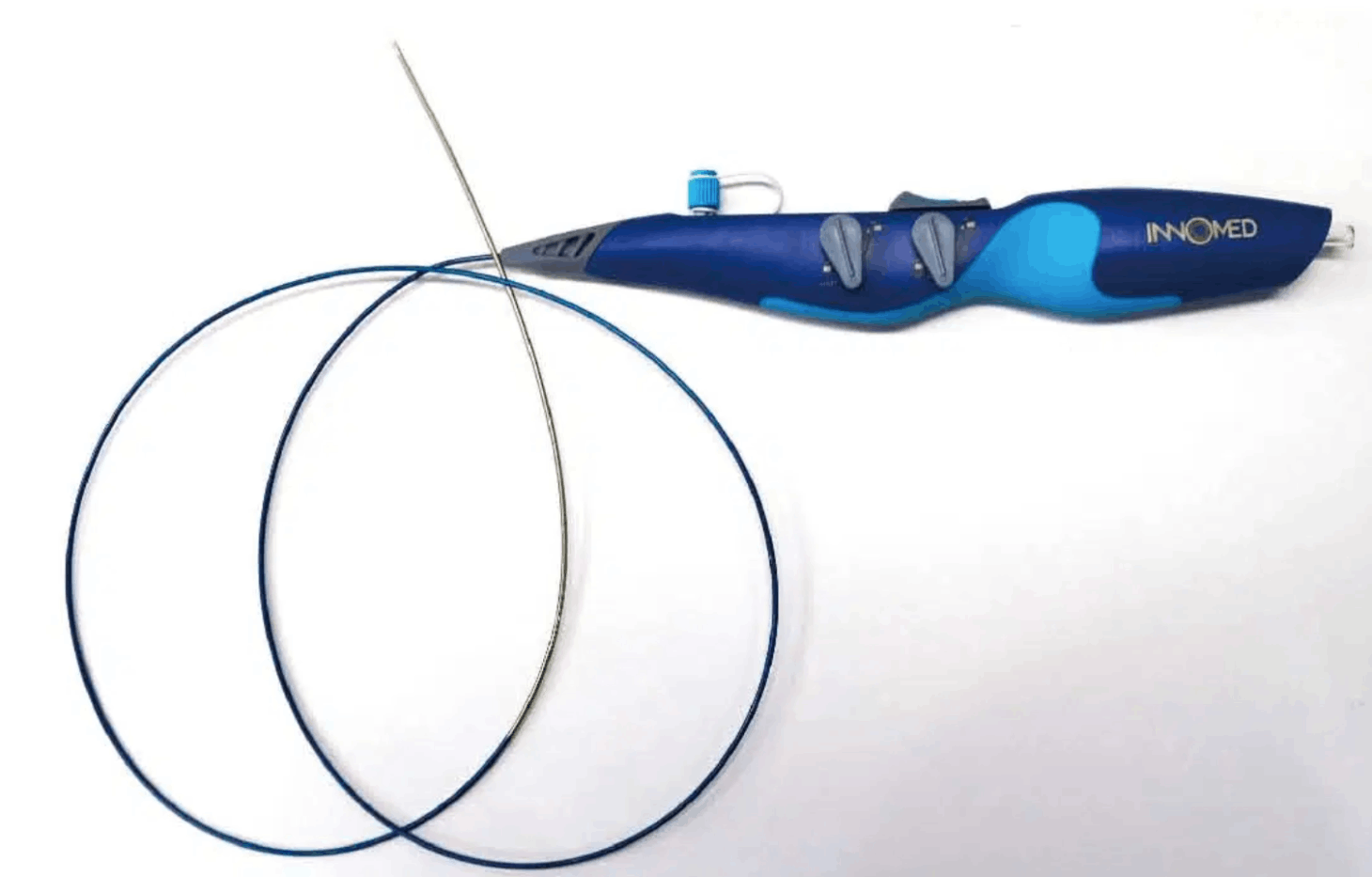
The iVAC 2L incorporates a patented rotating 2 way valve which is connected to an extra corporeal membrane pump via a 17Fr. single lumen, 100 cm long catheter. It can be used with any standard IABP console and does not require dedicated hardware.
When the heart is in the systolic phase, blood is aspirated from the left ventricle through the catheter tip and lumen into the membrane pump.
During the diastolic phase the membrane pump ejects the blood back through the catheter, subsequently opening the catheter valve and delivering the blood to the ascending aorta through the side outflow port, thereby creating an “extra beat of the heart”.
The pulsatile synchronization between the closing of the aortic valve and the opening of the catheter valve, ensures that aortic valve function is not impaired.
The iVAC 2L directly unloads the heart by active aspiration from the left ventricle, and simultaneously creates a counter pulsating flow in the ascending aorta.
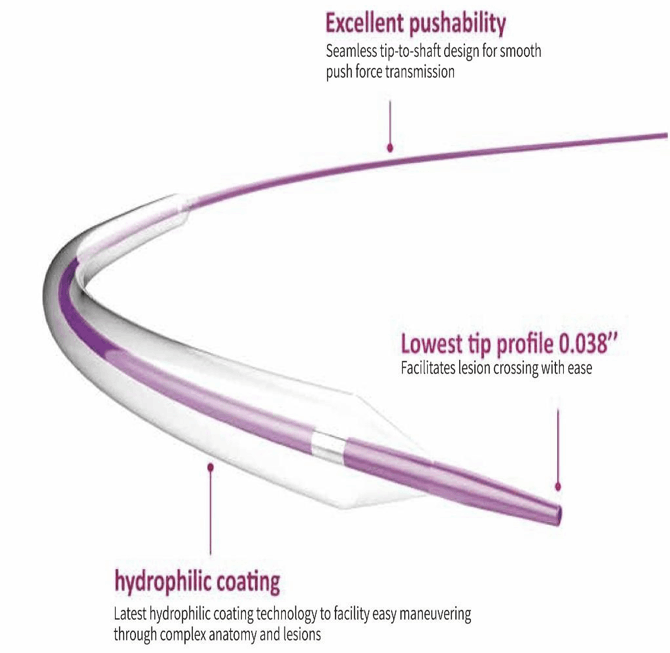
- Single lumen, 17Fr, 100 cm long catheter.
- Bi-directional flow catheter.
- ECG triggered counter pulsation.
- Provides pulsatile support.
- Increases mean arterial pressure.